Introduction
Aging is a natural process that affects every living organism. As we grow older, our bodies experience a decline in physiological functions, making us more susceptible to diseases and mortality. However, there are individuals who seem to defy the odds and live past the age of 100, exhibiting a delayed or escaped age-related decline. The study of these exceptional individuals, known as centenarians, has provided valuable insights into the genetic factors that influence the aging process.
In this article, we will explore the fascinating world of genetics and aging. We will delve into the genetic factors associated with longevity, the impact of DNA damage and telomeres on cell aging, and the role of specific genes in the aging process. Additionally, we will discuss the importance of longitudinal studies in understanding age-related decline within and among tissues. Finally, we will explore the emerging field of genomic convergence and how it is revolutionizing our understanding of human aging.
Genetic Factors in Longevity
One of the most intriguing aspects of human aging is the wide variation in lifespan among individuals. While environmental factors play a role in determining lifespan, genetic factors also contribute significantly. Studies comparing centenarians to average-aged individuals have identified several genetic factors associated with long life.
One well-known genetic factor is the APOE gene, which encodes the apolipoprotein E protein. Variants of this gene, such as the ɛ4 allele, have been associated with increased risk of Alzheimer’s disease and cardiovascular disease. Interestingly, individuals with the ɛ4 allele are less likely to live past the age of 100.
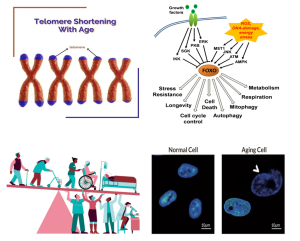
Another gene associated with longevity is FOXO3A, a transcription factor involved in the insulin/IGF-I signaling pathway.
Variants of this gene have been found to be enriched in centenarians of Asian and European populations. These variants may promote better health and contribute to extended lifespan by increasing the expression or activity of FOXO3A.
While these genetic factors have been replicated in multiple populations, they account for only a small percentage of the genetic contribution to longevity. It is clear that aging is a highly polygenic trait, influenced by a complex interplay of genetic and environmental factors.
The Role of DNA Damage and Telomeres in Aging
Cellular aging is characterized by the gradual accumulation of DNA damage and the shortening of telomeres, the protective caps at the ends of chromosomes. DNA damage can result from exposure to harmful environmental factors or deficiencies in DNA repair mechanisms. Progeroid syndromes, such as Werner syndrome and Hutchinson-Gilford progeria syndrome, are caused by mutations in genes involved in DNA repair and are characterized by accelerated aging symptoms.
Telomeres play a crucial role in maintaining chromosome stability and preventing chromosome instability. With each cell division, telomeres shorten, eventually leading to cell senescence, apoptosis, and an increased risk of mutation. Telomere shortening is associated with age-related diseases and accelerated aging syndromes. Conversely, telomerase, an enzyme that adds DNA sequence repeats to telomeres, can delay cell senescence and extend lifespan.
The relationship between DNA damage, telomere length, and aging is complex and not fully understood. However, it is clear that these factors contribute to the aging process and may serve as potential therapeutic targets for age-related diseases.
Longitudinal Studies of Human Aging
To gain a deeper understanding of the aging process, researchers have turned to longitudinal studies, which follow individuals over an extended period, collecting data on various age-related traits. These studies allow for the examination of age-related changes within and among tissues, providing valuable insights into the molecular basis of aging.
The Baltimore Longitudinal Study of Aging (BLSA) is one of the longest-running studies of human aging in the United States. Participants undergo comprehensive medical, physiological, and psychological assessments at regular intervals. The data collected from these individuals have been instrumental in identifying biomarkers of physiological age and uncovering genetic pathways involved in aging.
Another longitudinal study, the InCHIANTI study, focuses on the decline in mobility that occurs with age. This study includes residents of two small towns in Tuscany, Italy, and has collected data on a wide range of physiological measurements, including clinical histories, serum metabolite levels, and muscle strength levels.
By analyzing longitudinal data, researchers can identify genetic variants that predict how an individual will change over time. These variants may provide valuable insights into the molecular mechanisms underlying the aging process and can help identify potential targets for intervention.
Genomic Convergence: Combining Functional Genomic Information
Genomic convergence is an innovative approach that combines multiple types of functional genomic information, such as transcriptional profiling, gene association studies, and expression quantitative trait mapping. By integrating these diverse datasets, researchers can identify genes and pathways that are consistently associated with a specific phenotype.
One example of genomic convergence in the study of aging is the identification of the MMP20 gene in human kidney aging. Using gene expression data from kidney samples collected over a wide age range, researchers identified age-regulated genes and pathways associated with kidney aging.
The MMP20 gene, which encodes a matrix metalloproteinase involved in tissue remodeling, emerged as a potential candidate gene involved in the aging process.
Genomic convergence holds great promise for uncovering the complex genetic networks underlying aging and age-related diseases. By integrating multiple types of functional genomic data, researchers can gain a more comprehensive understanding of the molecular mechanisms driving the aging process.
Conclusion
Understanding the genetic factors that influence the aging process is a complex and evolving field of research. While certain genes, such as APOE and FOXO3A, have been consistently associated with longevity, they only account for a small fraction of the genetic contribution to aging. Longitudinal studies and genomic convergence approaches have provided valuable insights into the molecular basis of aging, identifying age-regulated genes, pathways, and potential therapeutic targets.
As our understanding of the genetics of aging continues to grow, we move closer to unlocking the secrets of longevity and improving the quality of life for individuals as they age. Understanding the genetics behind aging opens up new avenues for the development of anti-aging strategies and interventions. By targeting specific genetic pathways involved in the aging process, it may be possible to slow down or even reverse the effects of aging, leading to a healthier and longer life. The future holds exciting possibilities for unraveling the secrets of longevity and harnessing the power of genetics to enhance our quality of life as we age.
About Author
Anam Khan
Student of FY B. Sc.
Thakur College of Science & Commerce, Kandivali (E), Mumbai
Email- an24.02am@gmail.com
Leave a Reply